Gene Therapy Process Development Summit – What to Expect:
The inaugural Gene Therapy Process Development Summit is a must-attend to discover the latest insights to bridge the gap between R&D and process development, ensuring that high-quality gene therapies are manufactured repeatably, at scale, and in a cost-effective manner.
Join us in Philadelphia to discover and learn the latest insights through case study presentations, informative panel discussions, and deep-dive workshops from Ultragenyx, REGENXBIO, Janssen, Amgen, Astellas Gene Therapies, and many more!
Whether you are looking to improve your platform processes or shorten development timelines this summit will be a unique opportunity to meet and network with peers to discuss successes and challenges in your process development journeys.
In 2023 Your Peers had the opportunity to:
Learn how to achieve the ideal complex size with Meira GTx by using automation and process analytical tools to optimize transient transfection leading to enhanced efficiency, time saving and scale-up insights
Deep dive into enhancing the Pinnacle PCL Platform 2000L batch process with Ultragenyx to increase yield, improve product quality and reduce time-to-clinic
Examine case study results with Astellas Gene Therapies and explore the optimization techniques to cut costs and streamline tech transfer and scale-up times
Explore the design principles for creating robust AAV producer cell lines to yield a stable and consistent AAV production to optimize AAV vector generation to improve your platform processes with Lacerta and REGENXBIO
Unpick the ever-changing, complex regulations surrounding the world of gene therapy in a detailed workshop that doubles down on development strategies that are adaptable to the challenges by gene therapy to prevent regulatory roadblocks when trying to get drugs to clinic
Who attended?
The debut Gene Therapy Process Development Summit is dedicated to specifically addressing the highly technical upstream challenges of gene therapy in depth, attracting a niche audience of industry process development experts to foster meaningful conversations that create lasting, beneficial relationships in a rapidly evolving field.
This must-attend conference for biopharma process development, molecular biology, vector engineering, cell line development, MSAT and purification experts working in gene therapy will feature an in-depth regulatory workshop, dedicated networking opportunities, and detailed, technical case studies.
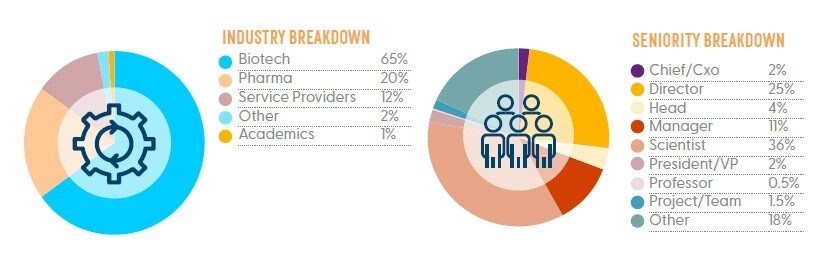
*Statistics based on Gene Therapy for Rare Disorders 2022
What Your Peers Have to Say:
“The conference provides information on all aspects of analytical development in the field of gene therapy and is a best place to meet key opinion leaders from the field.”
2022 attendee, NIB
“This is one of the best conferences I have ever attended as a scientist and vendor. The title is on point and extremely relevant. The audience was highly competent, motivated, and curious.”
2022 attendee, Malvern Panalytical
*Taken from Gene Therapy Analytical Development Summit 2022
Testimonials
“I am looking forward to networking with leading experts in the space to learn, and collectively discuss, novel strategies to overcome existing challenges.” – Gary Todd, Lacerta Therapeutics


“I am excited to attend a smaller, process development focussed gene therapy conference and interact with speakers and attendees from other relevant companies.” – Ping Liu, REGENXBIO
“I am looking forward to the opportunity to interact with fellow process development scientists, learn about exciting new applications in gene therapy and best practices for process intensification and clinical manufacturing.” – Steve Greenway, Vesigen Therapeutics


