Partnership Opportunities
With huge regulatory milestones such as the FDA approval of a gee therapy treatment for Spinal Muscular Atrophy and many more gene therapies headed towards later stage development and the continuous expansion of the gene therapy landscape, the need for repeatable and cost-effective process development is more important than ever.
The first Gene Therapy Process Development Summit will unite 60+ process development, CMC, MSAT and regulatory experts in Philadelphia. There are a limited number of partnership opportunities available, including speaking positions across our agenda. If you provide process development or vector engineering services and technologies that would benefit our niche industry audience, now is the time to secure new business and form connections with top biopharma companies seeking development solutions to progress their products to market.
Gene Therapy Process Development Experts Need Your Help With:
Upstream, downstream and analytical vector development platforms
Transfection reagents to deliver high AAV titers
Producing cGMP compliant and successful gene therapy vectors
Non-destructive assay providers to help develop stage appropriate potency assays to measure efficacy, expression and infectivity
Gene Therapy platform development providers
And more!
2023 Partners:

Why Partner?
Engage with a targeted audience of gene therapy process development experts from leading companies
Demonstrate your company's expertise and ability to meet the demands of the gene therapy field
Stand out from your competitors by showcasing your experience and innovative upstream technologies
Who Attended in 2023?
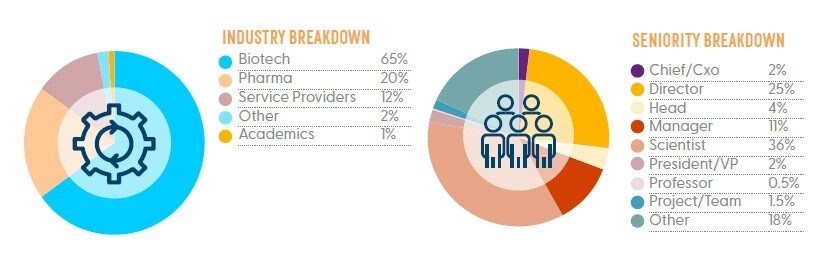
*Statistics based on Gene Therapy for Rare Disorders 2022

